Articles from CEL-SCI Corporation

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for three months ended December 31, 2025, as well as key recent clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · February 18, 2026

CEL-SCI Corporation (NYSE American: CVM) reported financial results for the fiscal year ended September 30, 2025, as well as key clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · December 29, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the closing of its offering of 1,111,200 shares of its common stock. Each share of common stock was sold at a public offering price of $9.00 per share. Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, were approximately $10 million.
By CEL-SCI Corporation · Via Business Wire · August 29, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the pricing of a best-efforts public offering of 1,111,200 shares of its common stock. Each share of common stock is being sold at a public offering price of $9.00 per share. Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, are expected to be approximately $10 million. The offering is expected to close on August 29, 2025, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · August 27, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced that it intends to offer to sell shares of its common stock (and/or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof) in a best-efforts public offering. All of the shares of common stock (and/or Pre-Funded Warrants) are being offered by the Company. The offering is subject to market conditions and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
By CEL-SCI Corporation · Via Business Wire · August 27, 2025

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for the three months ended June 30, 2025, as well as key recent clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · August 14, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced that a Breakthrough Medicine Designation application has been filed with the Saudi Food and Drug Authority (SFDA) for Multikine* (Leukocyte Interleukin, Injection) in the Kingdom of Saudi Arabia by one of the Kingdom’s premier pharmaceutical and healthcare companies. CEL-SCI has signed a Memorandum of Understanding (MOU) with this Saudi pharma company for the commercialization of Multikine in Saudi Arabia. A final partnership agreement is expected during the 3rd quarter of 2025.
By CEL-SCI Corporation · Via Business Wire · August 13, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the closing of its best-efforts offering of 1,500,000 shares of its common stock. Each share of common stock was sold at an offering price of $3.82 per share, priced at-the-market under NYSE American rules. Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, were approximately $5.7 million.
By CEL-SCI Corporation · Via Business Wire · July 14, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the pricing of a best-efforts offering of 1,500,000 shares of its common stock. Each share of common stock is being sold at an offering price of $3.82 per share, priced at-the-market under NYSE American rules. Total gross proceeds from the offering, before deducting the placement agent's fees and other offering expenses, are expected to be approximately $5.7 million. The offering is expected to close on July 14, 2025, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · July 11, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced it has reached an agreement with one of Saudi Arabia’s premier pharmaceutical and healthcare companies for a partnership that spans regulatory and commercial activities for Multikine* (Leukocyte Interleukin, Injection) in the Kingdom of Saudia Arabia. The formal agreement is expected to be signed with the Saudi pharmaceutical partner which will file a Breakthrough Medicine Designation application for Multikine with the Saudi Food and Drug Authority (SFDA) in the coming weeks. According to the SFDA, the response time to a Breakthrough Medicine Designation application is approximately 60 days. Following the granting of the Breakthrough Medicine Designation, Multikine would immediately become available for patient access and reimbursement/sale in Saudi Arabia. Several leading Saudi funds have expressed interest in investing in Multikine, CEL-SCI, and/or a potential joint venture to serve the wider Middle East and North Africa (MENA) market.
By CEL-SCI Corporation · Via Business Wire · July 11, 2025

CEL-SCI Corporation (NYSE American: CVM) today applauded the U.S. Food and Drug Administration’s (FDA) approval of Merck’s KEYTRUDA® (pembrolizumab), an anti-PD-1 therapy, for the treatment of adult patients with resectable locally advanced head and neck squamous cell carcinoma (HNSCC) whose tumors express PD-L1 (Combined Positive Score [CPS] ≥1) as determined by an FDA-approved test. Merck’s application was granted the FDA’s priority review on February 25, 2025, and regulatory approval was granted on June 13, 2025, based on interim results from Keytruda’s Phase 3 KEYNOTE-689 trial.
By CEL-SCI Corporation · Via Business Wire · June 18, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the closing of its underwritten public offering of 2,000,000 shares of its common stock at a public offering price of $2.50 per share. Total gross proceeds from the offering, before deducting the underwriting discount and other offering expenses, were $5,000,000. In addition, the Company has granted the underwriters a 45-day option to purchase up to an additional 190,000 shares to cover over-allotments at the public offering price, less the underwriting discount.
By CEL-SCI Corporation · Via Business Wire · May 23, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the pricing of an underwritten public offering of 2,000,000 shares of its common stock at a public offering price of $2.50 per share. Total gross proceeds from the offering, before deducting the underwriting discount and other offering expenses, are expected to be $5,000,000. In addition, the Company has granted the underwriters a 45-day option to purchase up to an additional 190,000 shares to cover over-allotments at the public offering price, less the underwriting discount. The offering is expected to close on May 23, 2025, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · May 21, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced that it intends to offer to sell shares of its common stock (and/or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof) in an underwritten public offering. All of the shares of common stock (and/or Pre-Funded Warrants) are being offered by the Company. The offering is subject to market conditions and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
By CEL-SCI Corporation · Via Business Wire · May 21, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced it has completed its Breakthrough Medicine Designation application for Multikine* (Leukocyte Interleukin, Injection) for submission to the Saudi Food and Drug Authority (SFDA) in Saudi Arabia. Multikine is an immunotherapy administered before surgery as a treatment for newly diagnosed previously untreated head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · May 21, 2025

CEL-SCI Corporation (NYSE American: CVM) announced today that during its annual Shareholder’s Meeting on May 19, 2025, a combination was authorized for its outstanding shares of common stock. On May 19, 2025, the Board of Directors approved a 1 for 30 combination of common stock. CEL-SCI expects the combination to be implemented on May 20, 2025. When the market opens on May 20, 2025, the common stock will trade under a new CUSIP number 150837 706, but the Company's ticker symbol, CVM, will remain unchanged.
By CEL-SCI Corporation · Via Business Wire · May 19, 2025

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for the three months ended March 31, 2025, as well as key recent clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · May 15, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced that it met with the Saudi Food and Drug Authority (SFDA) to discuss the development of Multikine cancer immunotherapy* (Leukocyte Interleukin, Injection), the vast amount of Multikine data available to support a marketing application for its use as a treatment of head and neck cancer, and the possible pathways to a marketing application in Saudi Arabia.
By CEL-SCI Corporation · Via Business Wire · April 23, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced that a study titled “Distinct CD8+ T cell dynamics associate with response to neoadjuvant cancer immunotherapies” by Li Housaiyin et. al., Cancer Cell (2025) provides support for CEL-SCI’s approach aimed at seeking early regulatory approval for Multikine* (Leukocyte Interleukin, Injection) as a neoadjuvant in the treatment of newly diagnosed previously untreated locally advanced head and neck cancer based on early tumor responses.
By CEL-SCI Corporation · Via Business Wire · April 8, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced new data has been published from its prior Phase 3 study of Multikine* (Leukocyte Interleukin, Injection) in newly diagnosed, treatment naïve, resectable, locally advanced head and neck cancer patients in the highly regarded peer reviewed journal Pathology and Oncology Research (POR). The article titled “Neoadjuvant Leukocyte Interleukin Injection Immunotherapy Improves Overall Survival in Low-risk Locally Advanced Head and Neck Squamous Cell Carcinoma -The IT-MATTERS Study” included a comprehensive presentation of results from CEL-SCI’s Phase 3 trial, the largest study ever conducted for newly diagnosed locally advanced head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · March 24, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the closing of its previously announced best-efforts offering of 16,000,000 shares of its common stock (or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof). Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, is approximately $2,560,000. All the shares and Pre-Funded Warrants in the offering were offered by the Company
By CEL-SCI Corporation · Via Business Wire · March 18, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the pricing of a best-efforts offering of 16,000,000 shares of its common stock (or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof). Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, are expected to be approximately $2,560,000. The offering is expected to close on March 18, 2025, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · March 17, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced it received comments from the U.S. Food and Drug Administration (FDA) on the confirmatory Registration Study’s Statistical Analysis Plan (SAP) submitted in December of 2024 for the study of Multikine* (Leukocyte Interleukin, Injection) as a neoadjuvant in the treatment of newly diagnosed previously untreated locally advanced head and neck cancer. The FDA stated no response to their comments were required from CEL-SCI and that the agency presently has no comments on the confirmatory study protocol, which was submitted for FDA review contemporaneously with the SAP in December 2024.
By CEL-SCI Corporation · Via Business Wire · March 17, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced that a third-party study published on March 6, 2025 in JAMA Oncology titled “Neoadjuvant Nivolumab Plus Chemotherapy Followed by Response-Stratified Chemoradiation Therapy in HPV-Negative Head and Neck Cancer: The DEPEND Phase 2 Non-randomized Clinical Trial” provided data that support Multikine’s use as a neoadjuvant treatment in patients with tumors having low PD-L1 expression in its upcoming confirmatory head and neck cancer Registration Trial.
By CEL-SCI Corporation · Via Business Wire · March 14, 2025

CEL-SCI Corporation (NYSE American: CVM) today announced it is in the final stages for the launch of its 212-patient Confirmatory Registration Study for Multikine* (Leukocyte Interleukin, Injection) in newly diagnosed locally advanced head and neck cancer patients. This final Registration Study is specifically designed to confirm the statistically significant efficacy and safety results from CEL-SCI’s previously completed randomized controlled Phase 3 trial.
By CEL-SCI Corporation · Via Business Wire · February 20, 2025

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for three months ended December 31, 2024, as well as key recent clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · February 18, 2025

CEL-SCI Corporation (NYSE American: CVM) reported financial results for the fiscal year ended September 30, 2024, as well as key clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · January 14, 2025

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a cancer immunotherapy company, today announced the closing of its previously announced best-efforts offering of 16,130,000 shares of its common stock (or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof). Each share of common stock (or Pre-Funded Warrant) was sold at an offering price of $0.31 per share (inclusive of the Pre-Funded Warrant exercise price), for gross proceeds of approximately $5,000,000, before deducting placement agent fees and other offering expenses. All the shares and Pre-Funded Warrants in the offering were offered by the Company.
By CEL-SCI Corporation · Via Business Wire · December 31, 2024

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the pricing of a best-efforts public offering of 16,130,000 shares of its common stock (or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof). Each share of common stock (or Pre-Funded Warrant) is being sold at a public offering price of $0.31 per share (inclusive of the Pre-Funded Warrant exercise price). Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, are expected to be approximately $5,000,000. The offering is expected to close on December 31, 2024, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · December 29, 2024

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced that it intends to offer to sell shares of its common stock (and/or pre-funded warrants in lieu thereof) in a best efforts public offering. The offering is subject to market conditions and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
By CEL-SCI Corporation · Via Business Wire · December 27, 2024

CEL-SCI Corporation (NYSE American: CVM) today highlights strong biological rationale for the use of Multikine in the confirmatory registration head and neck cancer study. This study of 212 newly diagnosed locally advanced, resectable head and neck cancer patients was given the go-ahead as a confirmatory registration study by FDA and will focus on those patients who showed a 73% survival with Multikine vs. a 45% for the control patients not treated with Multikine in the prior Phase 3 study.
By CEL-SCI Corporation · Via Business Wire · December 12, 2024

CEL-SCI Corporation (NYSE American: CVM) today announced that in a recent meeting the U.S. Food and Drug Administration (FDA) concurred with the Company’s approach to patient selection using low PD-L1 tumor expression in its confirmatory Registration Study for Multikine® (Leukocyte Interleukin, Injection)*. This study will focus on the treatment of newly diagnosed locally advanced primary head and neck cancer patients with no lymph node involvement and low (TPS <10) PD-L1 tumor expression. This Registration Study, slated to commence in the first quarter of 2025, will enroll approximately 212 patients and prospectively confirm the favorable safety profile and the very favorable efficacy results demonstrated in the target population in CEL-SCI’s prior Phase 3 randomized study of 928 patients.
By CEL-SCI Corporation · Via Business Wire · November 7, 2024

CEL-SCI Corporation (NYSE American: CVM) today announced the potential positive impact on the clinical development of its immunotherapy Multikine® (Leukocyte Interleukin, Injection)* resulting from a recent U.S. Food and Drug Administration (FDA) Oncologic Drugs Advisory Committee (ODAC) meeting, a public forum.
By CEL-SCI Corporation · Via Business Wire · October 22, 2024

CEL-SCI Corporation (NYSE American: CVM) today announced its renewed collaboration with Ergomed Clinical Research for its upcoming U.S. Food and Drug Administration (FDA) confirmatory Registration Study of Multikine® (Leukocyte Interleukin, Injection) in head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · October 1, 2024
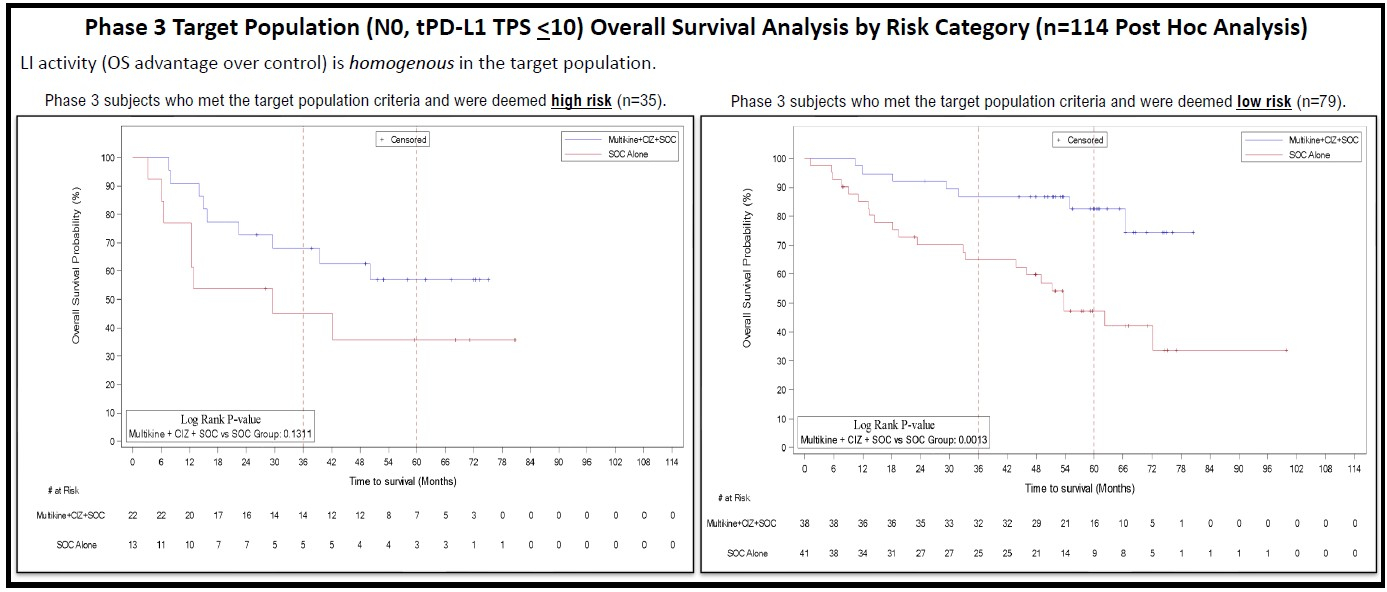
CEL-SCI Corporation (NYSE American: CVM) today reported new data from its concluded Phase 3 study of Multikine® (Leukocyte Interleukin, Injection)* that were presented at the European Society for Medical Oncology (ESMO) 2024 Congress on Saturday, September 14, 2024 in a poster titled “Prognostic significance of diagnostic staging in treatment naïve, resectable locally advanced primary oral cavity squamous cell carcinoma for neoadjuvant Leukocyte Interleukin Injection immunotherapy”. This data is highly relevant to CEL-SCI’s 212 patient confirmatory Registration Study which has received the U.S. Food and Drug Administration’s (FDA) go-ahead and is currently under preparation.
By CEL-SCI Corporation · Via Business Wire · September 16, 2024

CEL-SCI Corporation (NYSE American: CVM) today reported it will report new data from its Phase 3 study of Multikine (Leukocyte Interleukin, Injection)* at the European Society for Medical Oncology (ESMO) 2024 Congress which takes place from September 13 – 17, 2024 in Barcelona, Spain. A poster titled “Prognostic significance of diagnostic staging in treatment naïve, resectable locally advanced primary oral cavity squamous cell carcinoma for neoadjuvant Leukocyte Interleukin Injection immunotherapy” will be presented by the study’s co-author József Tímár MD, PhD, DSc, a prominent and highly respected pathologist.
By CEL-SCI Corporation · Via Business Wire · September 10, 2024

CEL-SCI Corporation (NYSE American: CVM) today reported it has received a decision letter from the United Kingdom’s Healthcare Products Regulatory Agency (MHRA) granting Multikine (Leukocyte Interleukin, Injection)* a product specific waiver for the treatment of head and neck cancer in a pediatric population of people up to 18 years of age. As a result, CEL-SCI will not be required to evaluate Multikine in a pediatric population as part of license and marketing clearance review in the UK.
By CEL-SCI Corporation · Via Business Wire · September 4, 2024
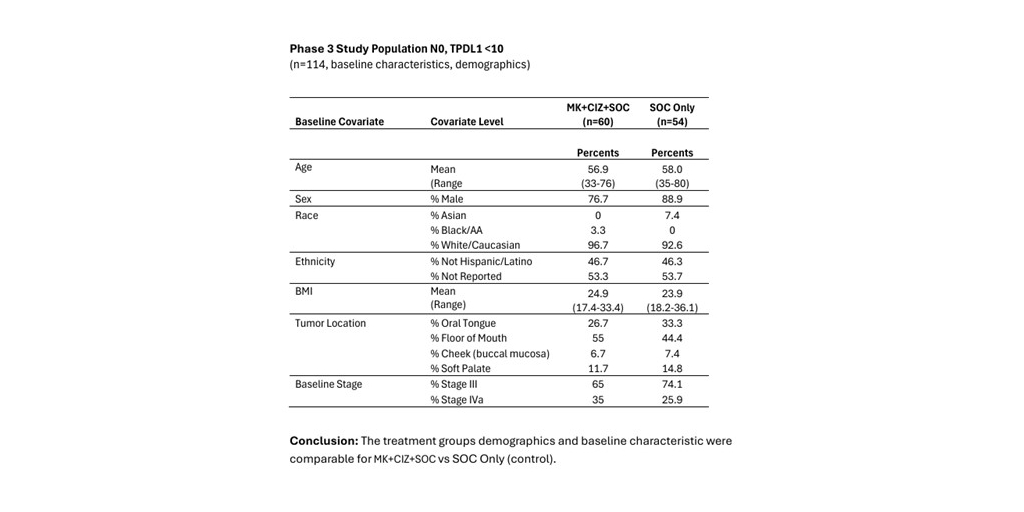
CEL-SCI Corporation (NYSE American: CVM) today reported financial results for the quarter ended June 30, 2024, as well as key recent clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · August 15, 2024

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a cancer immunotherapy company, today announced the closing of its previously announced best-efforts offering of 10,845,000 shares of its common stock (or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof). Each share of common stock (or Pre-Funded Warrant) was sold at an offering price of $1.00 per share (inclusive of the Pre-Funded Warrant exercise price), for gross proceeds of $10,845,000, before deducting placement agent fees and other offering expenses. All the shares and Pre-Funded Warrants in the offering were offered by the Company.
By CEL-SCI Corporation · Via Business Wire · July 29, 2024

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a cancer immunotherapy company, today announced the pricing of a best-efforts offering of 10,845,000 shares of its common stock (or pre-funded warrants (“Pre-Funded Warrants”) in lieu thereof). Each share of common stock (or Pre-Funded Warrant) is being sold at an offering price of $1.00 per share (inclusive of the Pre-Funded Warrant exercise price). All of the shares and Pre-Funded Warrants in the offering are being offered by the Company. Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, are expected to be $10,845,000. The offering is expected to close on July 29, 2024, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · July 26, 2024
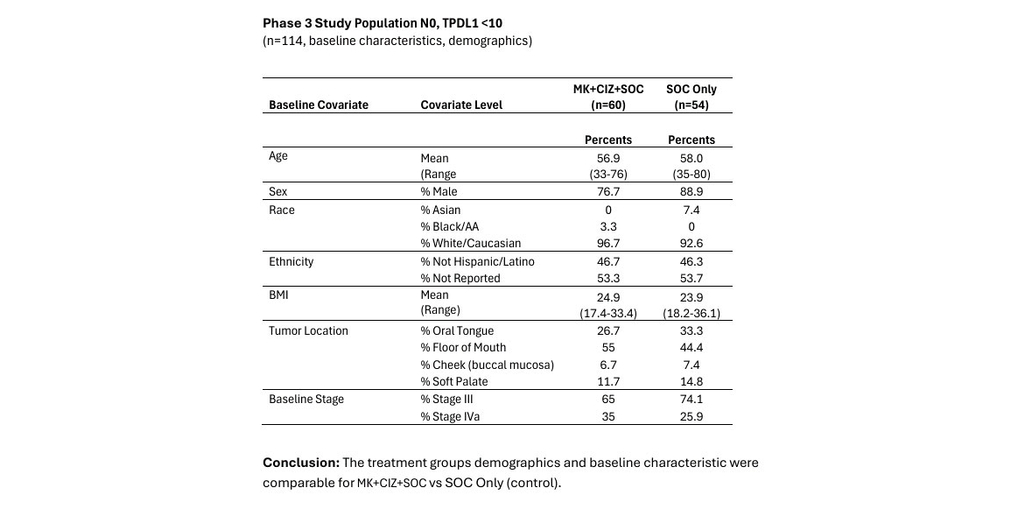
CEL-SCI Corporation (NYSE American: CVM) today reported positive results from a bias analysis conducted for its concluded Phase 3 study of Multikine (Leukocyte Interleukin, Injection)* in the treatment of head and neck cancer. Conducting a bias analysis is a standard process used to identify, assess, and address potential sources of bias that could influence the outcomes and interpretations of study results. The goal of a bias analysis is to ensure that the trial's findings are reliable, the conclusions are valid, and to minimize the risk that bias has distorted the results.
By CEL-SCI Corporation · Via Business Wire · July 26, 2024

CEL-SCI Corporation (NYSE American: CVM) today announced that Robert (“Bob”) Watson, who has served as a Director of the Company since 2017, has been appointed Chairperson of the Board.
By CEL-SCI Corporation · Via Business Wire · July 8, 2024
CEL-SCI Corporation (NYSE American: CVM) today announced the Company’s Chief Scientific Officer, Dr. Eyal Talor, delivered a presentation titled “Neoadjuvant Immunotherapy for Head and Neck Cancer: Low Tumor PD-L1 Expression - IT-MATTERS – RCT” at the International Drug Discovery Science & Technology (IDDST) 20th Annual Congress in Budapest, Hungary on Tuesday, June 18, 2024. Dr. Talor presented during the Cancers/Tumors session which he Chaired along with Dr. Elizabeth Tran of Purdue University.
By CEL-SCI Corporation · Via Business Wire · June 18, 2024
CEL-SCI Corporation (NYSE American: CVM) today announced Dr. Giovanni Selvaggi, an oncology key opinion leader instrumental in successfully bringing several drugs to market has joined CEL-SCI as a Clinical Advisor. Dr. Selvaggi joins CEL-SCI as the Company recently received its go-ahead from the U.S. Food and Drug Administration (FDA) for its confirmatory Registration Study of Multikine* in the treatment of head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · June 6, 2024

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for the quarter ended March 31, 2024, as well as key recent clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · May 16, 2024

CEL-SCI Corporation (NYSE American: CVM) today announced a significantly positive outcome from its recent meeting with the U.S. Food and Drug Administration (FDA) regarding the path to approval for its first-line investigational cancer immunotherapy Multikine* (Leukocyte Interleukin, Injection). Based on strong safety and efficacy data from CEL-SCI’s completed Phase 3 head and neck cancer study, the FDA indicated CEL-SCI may move forward with a confirmatory Registration Study of Multikine in newly diagnosed advanced primary head and neck cancer patients with no lymph node involvement (determined via PET scan) and with low PD-L1 tumor expression (determined via biopsy).
By CEL-SCI Corporation · Via Business Wire · May 8, 2024

CEL-SCI Corporation (NYSE American: CVM) today announced the appointment of Mario Gobbo to its Board of Directors.
By CEL-SCI Corporation · Via Business Wire · April 23, 2024

CEL-SCI Corporation (NYSE American: CVM) today announced the peer reviewed scientific journal Frontiers in Immunology published an article regarding CEL-SCI’s Ligand Epitope Antigen Presentation System (LEAPS) technology in the treatment of rheumatoid arthritis (RA) titled: “Current status of immunological therapies for rheumatoid arthritis with a focus on antigen-specific therapeutic vaccines”. CEL-SCI’s LEAPS team, along with outside collaborators from U.S. and Europe, reviewed published/presented results from pre-clinical animal model of antigen-specific LEAPS therapeutic vaccines, such as CEL-4000 and CEL-5000, made specific for the treatment of RA, and compared these findings with published results of other RA-therapies that either suppress or alter the immune response in order to treat RA.
By CEL-SCI Corporation · Via Business Wire · March 19, 2024

CEL-SCI Corporation (NYSE American: CVM) today issued a letter to its shareholders. This letter will be sent to the Company’s shareholders along with the proxy to the upcoming annual meeting.
By CEL-SCI Corporation · Via Business Wire · March 6, 2024

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for the quarter ended December 31, 2023, as well as key recent clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · February 15, 2024

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the closing of its previously announced public offering of 3,875,000 shares of its common stock at a public offering price of $2.00 per share, for gross proceeds of $7.75 million, before deducting underwriting discounts and offering expenses. All of the shares of common stock are being offered by the Company.
By CEL-SCI Corporation · Via Business Wire · February 13, 2024

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the pricing of an offering of 3,875,000 shares of its common stock at an offering price of $2.00 per share, for gross proceeds of $7.75 million, before deducting underwriting discounts and offering expenses. All of the shares of common stock are being offered by the Company. The offering is expected to close on February 13, 2024, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · February 9, 2024

CEL-SCI Corporation (NYSE American: CVM) today reported that its Multikine® (Leukocyte Interleukin, Injection)* cGMP state-of-the-art dedicated manufacturing facility commissioning has been completed.
By CEL-SCI Corporation · Via Business Wire · February 6, 2024
CEL-SCI Corporation (NYSE American: CVM) today announced that the European Medicines Agency (EMA) Paediatric Committee granted CEL-SCI a product-specific waiver of strict requirements for commercialization of cancer drugs in the European Union (EU). According to the opinion letter:
By CEL-SCI Corporation · Via Business Wire · January 31, 2024

CEL-SCI Corporation (NYSE American: CVM) reported financial results for the fiscal year ended September 30, 2023, as well as key clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · December 22, 2023

CEL-SCI Corporation (NYSE American: CVM) today announced that the British National Institute for Health and Care Excellence (NICE) has selected Multikine* (Leukocyte Interleukin, Injection) to be evaluated as the potential new standard of care for squamous cell carcinoma of the head and neck (SCCHN) in the UK. NICE posted a detailed report from the UK’s National Institute for Health and Care Research (NIHR) regarding Multikine, its clinical data, and its potential to become a better standard of care in treating newly diagnosed head and neck cancer in the UK. This published report informs UK doctors, patients, and other interested parties that NICE has started the review of Multikine and is soliciting public comment.
By CEL-SCI Corporation · Via Business Wire · December 4, 2023

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the closing of its previously announced public offering of 2,490,000 shares of its common stock to a single investor at a public offering price of $2.00 per share, for gross proceeds of approximately $5 million, before deducting underwriting discounts and offering expenses. All of the shares of common stock were offered by the Company.
By CEL-SCI Corporation · Via Business Wire · November 20, 2023

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the pricing of an offering of 2,490,000 shares of its common stock to a single investor at an offering price of $2.00 per share, for gross proceeds of approximately $5 million, before deducting underwriting discounts and offering expenses. All of the shares of common stock are being offered by the Company. The offering is expected to close on November 20, 2023, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · November 16, 2023

CEL-SCI Corporation (NYSE American: CVM) today released a letter to shareholders from the Company’s CEO, Geert Kersten. The very comprehensive letter details the data reported on the efficacy of Multikine (Leukocyte Interleukin, Injection)* in the head and neck cancer target patient population as well as CEL-SCI’s plan to file for immediate regulatory approval. The shareholder letter can be read in full on the Company’s website (https://cel-sci.com/) or by clicking HERE.
By CEL-SCI Corporation · Via Business Wire · October 30, 2023

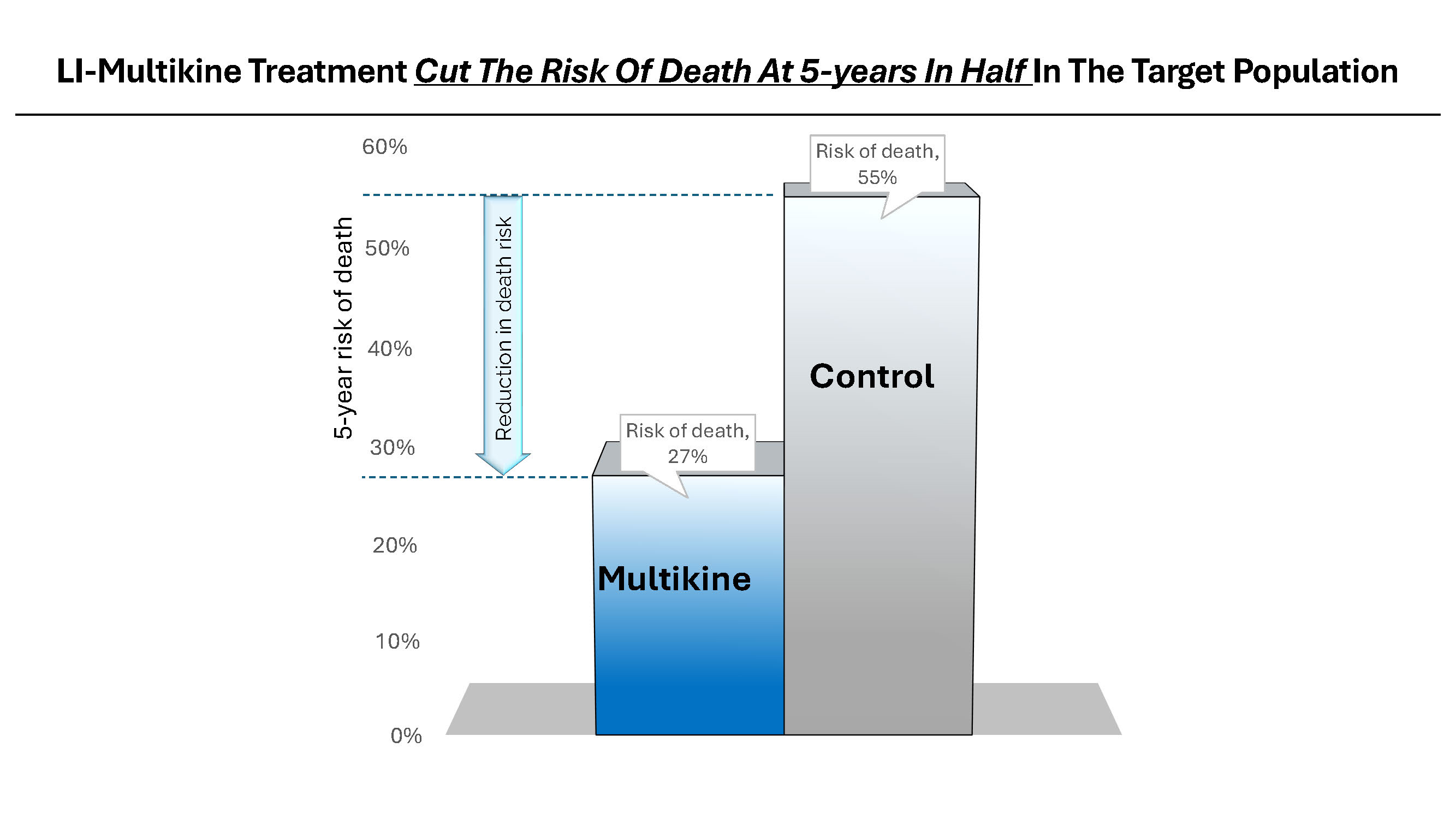
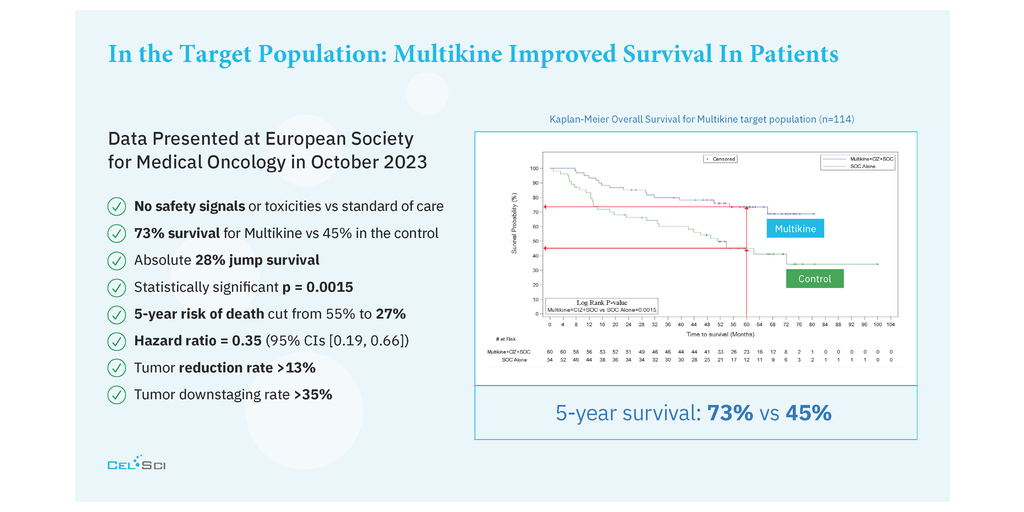
CEL-SCI Corporation (NYSE American: CVM) today released a video presentation in which the Company’s CEO, Geert Kersten, shares the most recent data presented at the European Society for Medical Oncology (ESMO) Congress and the data’s impact on propelling the Company’s immunotherapy drug Multikine* (Leukocyte Interleukin, Injection) toward regulatory approval for the treatment of newly diagnosed, advanced squamous cell carcinoma of the head and neck (SCCHN). Mr. Kersten carefully explains how it is that patients in the target group who were treated with Multikine had a 5-year survival rate of 73% as compared to only 45% for those who did not receive Multikine, cutting the risk of death by half. He goes on to present CEL-SCI’s regulatory submissions plan and timelines based on these compelling findings for a patient population that has not had a new treatment approved in in the U.S. in many decades.
By CEL-SCI Corporation · Via Business Wire · October 24, 2023
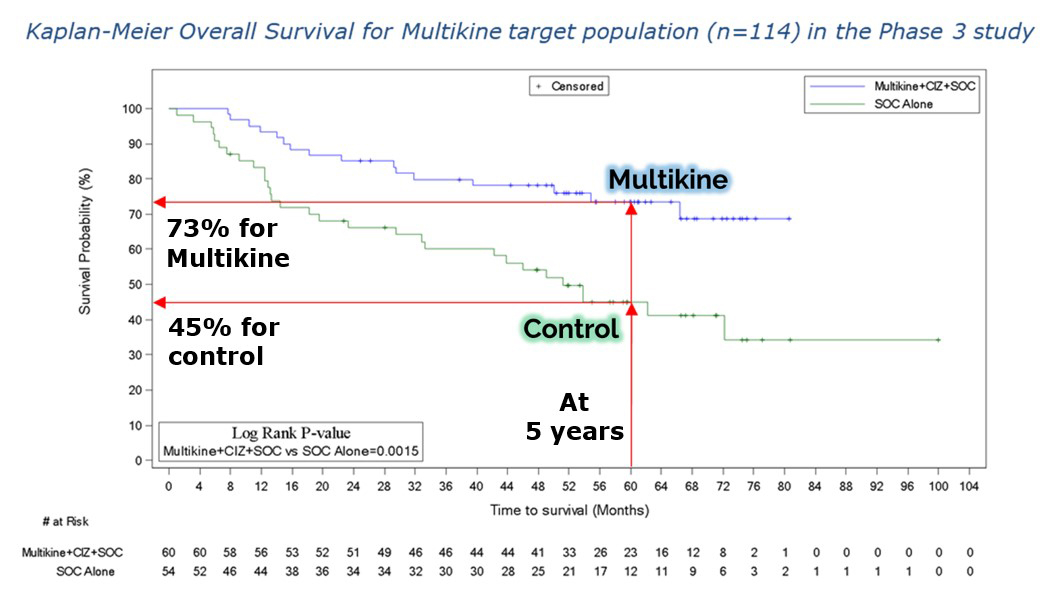
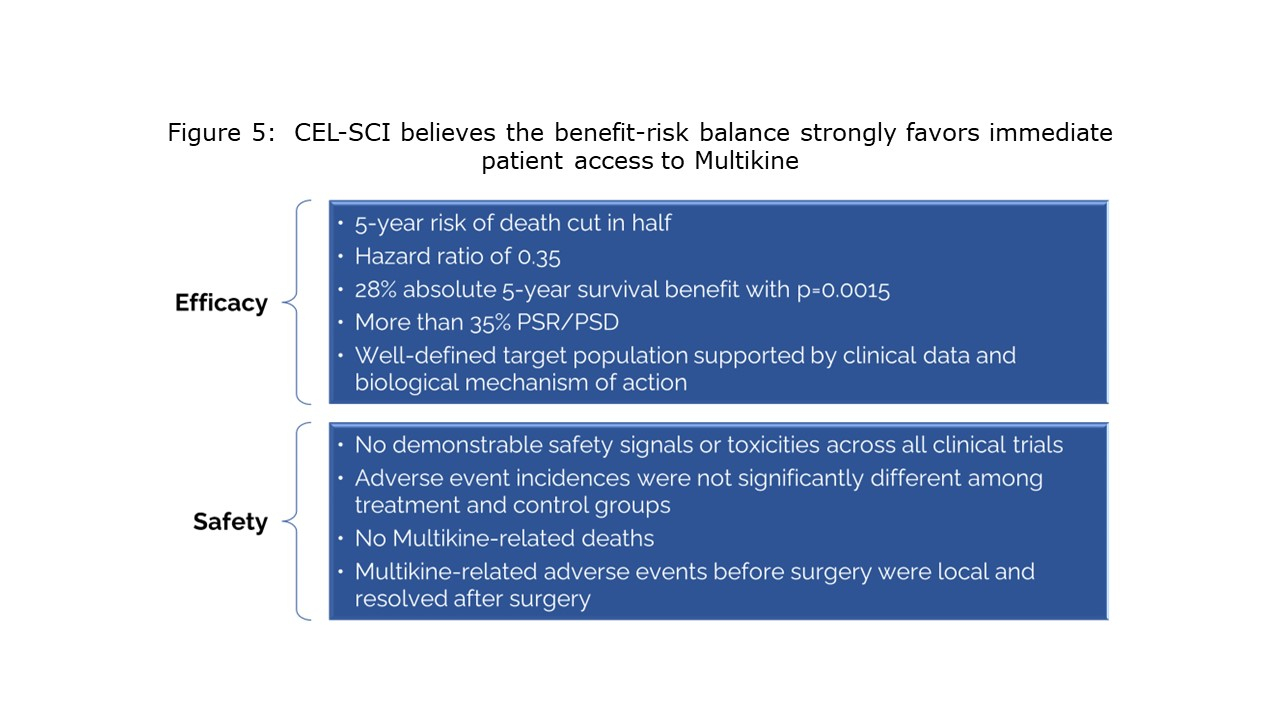
CEL-SCI Corporation (NYSE American: CVM) today announced that it has finalized the selection criteria for the head and neck cancer target population to be treated with the Company’s immunotherapy drug Multikine (Leukocyte Interleukin, Injection). Five-year survival in the target population was 73% alive for Multikine-treated patients vs only 45% alive in the control who did not receive Multikine, with the five-year risk of death cut in half for Multikine-treated subjects in the target population versus the control. These data, which are statistically significant and accompanied by strong hazard ratios, are a crucial achievement on the path for the approval of Multikine. CEL-SCI presented the data for the first time at the European Society for Medical Oncology (ESMO) Congress in Spain on October 22, 2023. The selection criteria for this target population were developed based on the completed Phase 3 randomized controlled trial, advice from regulators, and advice from physician consultants associated with the University of California San Diego Cancer Center and Yale Medical School, recognized as among the nation’s most esteemed immuno-oncologists in head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · October 23, 2023

CEL-SCI Corporation (NYSE American: CVM) today reported that its Multikine* (Leukocyte Interleukin, Injection) cGMP state-of-the-art dedicated manufacturing facility commissioning is substantially complete, a significant milestone toward a planned Biologics License Application (BLA) with several regulatory agencies for approval of Multikine in the treatment of head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · October 19, 2023

CEL-SCI Corporation (NYSE American: CVM) today reported it has filed a request with the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) to discuss a pathway for approval of Multikine* (Leukocyte Interleukin, Injection) immunotherapy for the treatment of newly diagnosed head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · October 5, 2023

CEL-SCI Corporation (NYSE American: CVM) today reported it has filed a request for Scientific Advice regarding Multikine* (Leukocyte Interleukin, Injection) immunotherapy for the treatment of newly diagnosed locally advanced squamous cell carcinoma of the head and neck (SCCHN) with the European Medicines Agency’s (EMA’s) Scientific Advice Working Group.
By CEL-SCI Corporation · Via Business Wire · September 26, 2023

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for the quarter ended June 30, 2023, as well as key clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · August 11, 2023

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the closing of its previously announced public offering of 2,500,000 shares of its common stock at a public offering price of $2.00 per share, for gross proceeds of $5,000,000, before deducting underwriting discounts and offering expenses.
By CEL-SCI Corporation · Via Business Wire · July 20, 2023

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the pricing of an underwritten public offering of 2,500,000 shares of its common stock at a public offering price of $2.00 per share, for gross proceeds of $5,000,000, before deducting underwriting discounts, and offering expenses. All of the shares of common stock are being offered by the Company. The offering is expected to close on July 20, 2023, subject to satisfaction of customary closing conditions.
By CEL-SCI Corporation · Via Business Wire · July 17, 2023

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced that it intends to offer to sell shares of its common stock in an underwritten public offering. The Company expects to grant the underwriter a 45-day option to purchase up to an additional 15% of the number of shares of common stock sold in this offering to cover over-allotments, if any. All of the shares of common stock are being offered by the Company. The offering is subject to market conditions and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
By CEL-SCI Corporation · Via Business Wire · July 17, 2023

CEL-SCI Corporation (NYSE American: CVM) today announced it has concluded a productive meeting with the U.S. Food and Drug Administration (FDA) regarding the path forward for bringing Multikine* (Leukocyte Interleukin, Injection) immunotherapy to market for the treatment of newly diagnosed locally advanced squamous cell carcinoma of the head and neck (SCCHN). During the recent meeting, the FDA acknowledged the longstanding need for improved treatments for head and neck cancer.
By CEL-SCI Corporation · Via Business Wire · July 14, 2023

CEL-SCI Corporation (NYSE American: CVM) today announced new data from a biomarker analysis of its pivotal Phase 3 study in newly diagnosed locally advanced squamous cell carcinoma of the head and neck (SCCHN) at the American Head and Neck Cancer Society’s (AHNS) 11th Annual International Conference on Head and Neck Cancer on July 10, 2023 in Montreal, Canada, in the presentation titled “Tumor cell PD-L1 biomarker confirms Leukocyte Interleukin Injection (LI) treatment (Tx) survival outcome advantage in naïve locally advanced primary head & neck squamous cell carcinoma (SCCHN), the IT-MATTERS Study”. The Leukocyte Interleukin Injection (LI) [aka Multikine*] talk, was delivered by Philip Lavin, PhD, lead biostatistician for over 80 regulatory approvals/clearances who has also served on multiple U.S. Food and Drug Administration (FDA) review panels. Dr. Lavin is the lead biostatistician for CEL-SCI’s IT-MATTERS study.
By CEL-SCI Corporation · Via Business Wire · July 11, 2023

CEL-SCI Corporation (NYSE American: CVM) today announced it will present the new data from its pivotal Phase 3 study, the largest study ever conducted in newly diagnosed locally advanced squamous cell carcinoma of the head and neck (SCCHN), at the American Head and Neck Cancer Society’s (AHNS) 11th Annual International Conference on Head and Neck Cancer on July 8-12, 2023 in Montreal, Canada.
By CEL-SCI Corporation · Via Business Wire · June 22, 2023

CEL-SCI Corporation (NYSE American: CVM) today announced that the latest results from its pivotal Phase 3 study of Multikine, a presurgical cancer immunotherapy, were presented at the European Society for Radiotherapy and Oncology (ESTRO) 2023 Congress in Vienna, Austria in a poster presentation titled “Histopathology population (HPP) confirms Multikine* [Leukocyte Interleukin Injection (LI)] treatment (Tx) outcome in naïve locally advanced primary head & neck squamous cell carcinoma SCCHN)” during the Head & Neck Cancer Session on Saturday, May 13, 2023.
By CEL-SCI Corporation · Via Business Wire · May 15, 2023

CEL-SCI Corporation (NYSE American: CVM) today reported financial results for the quarter ended March 31, 2023, as well as key clinical and corporate developments.
By CEL-SCI Corporation · Via Business Wire · May 12, 2023

CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a Phase 3 cancer immunotherapy company, today announced the pricing of its underwritten confidentially marketed public offering of 794,117 shares of common stock at an offering price of $1.70 per share. The closing of the offering is expected to take place on or about May 2, 2023, subject to the satisfaction of customary closing conditions. In addition, the Company expects to grant the underwriter a 30-day option to purchase up to an additional 15 percent of the shares of common stock to cover over-allotments.
By CEL-SCI Corporation · Via Business Wire · April 27, 2023

CEL-SCI Corporation (NYSE American: CVM) today announced it has held a productive pre-submission meeting with Canada’s regulator to determine the best regulatory path toward market approval. Based on the existing data that was summarized and presented, Health Canada advised CEL-SCI to request advance consideration for approval under a Notice of Compliance with Conditions policy. Additional discussions explored how patients at lower risk for recurrence could be targeted for treatment, and what sort of post-market commitments could help ensure that only the most suitable patients would be treated with Multikine®* (Leukocyte Interleukin Injection), based on the best available evidence.
By CEL-SCI Corporation · Via Business Wire · April 19, 2023
